Artificial Disc Replacement
1. What is artificial disc replacement?
Traditionally spinal surgeons have performed fusion surgery for painful neck and back conditions. Fusion is essentially a welding process. The basic idea is to fuse together the affected vertebrae so that they heal into a single, solid bone, eliminating painful motion. While many patients are helped by spinal fusion, the results of the surgery can vary. In addition, some patients whose fusion surgeries heal perfectly still end up with no improvement of their pain. Some doctors believe that the failure to improve after fusion surgery is due to the fact that fusion prevents normal motion in the spine. For this reason, artificial disk replacement — which aims to preserve normal motion — has emerged as an alternative treatment option.
Artificial disk replacement initially gained FDA approval for use in the U.S. in 2004. Since then, numerous disk replacement designs have been developed, and more are currently being tested.
Worn or damaged disk material between the small bones in the spine (vertebrae) is removed and replaced with a prosthetic, or artificial disk. The goal of the procedure is to relieve pain while maintaining more normal motion than is allowed with some other procedures, such as spinal fusion (which aims to prevent motion).
2. Am I a candidate for disc replacement?
Artificial disk replacement is not appropriate for all patients with spinal pain. In general, good candidates for disk replacement have the following characteristics:
- Pain caused by one or two problematic (arthritic) intervertebral disks in the spine
- Nerve pain radiating in to the arm (cervical disc replacement i.e. neck)
- Back pain arising from a damaged disc (lumbar disc replacement i.e. back)
- No significant facet joint disease
- Body size that is not excessively overweight (lumbar)
- No prior major surgery on the abdomen (lumbar)
- No deformity of the spine (scoliosis)
- No osteoporosis (weak bone)
- No infection
- No instability
3. Testing & Diagnosis
You will have a thorough evaluation to try and determine the primary cause of neck or back pain or radiculopathy. This includes a detailed history and physical exam, which may focus on which body positions cause or reproduce pain.
Tests include x-rays, magnetic resonance imaging (MRI) and computed tomography (CT) and CT SPECT. Most often, prior to recommending surgery, we need to assess the patient’s bones and discs with at least two of these imaging techniques. On occasion there might be a need to order a provocative discogram (lumbar only) to help decide if the worn or damaged disc seen on imaging is the cause of the pain. For lumbar disc replacement, you will be asked to consult with an access Vascular surgeon who helps the spinal surgeon with accessing the front of the spine through an abdominal incision.
4. Alternative Treatment options prior to considering surgery
Non-surgical treatment options for neck pain, radiculopathy and discogenic back pain include medications to relieve pain, physical therapy, spinal injections, bracing, alternative therapies and lifestyle modifications (smoking cessation, diet, weight loss, exercise). Pain management with a pain specialist may be advised.
5. Types of disc replacement devices
Artificial disc replacement prosthesis come in many different shapes and sizes, but current designs fall into one of four types: hydraulic, elastic, and mechanical discs.
- Composite – A composite artificial disc is comprised of several pieces, often two metal endplates with a polyethylene (plastic) spacer sandwiched in between them.
- Hydraulic – A hydraulic artificial disc contains a dehydrated core that is implanted in a compressed state. The saline-hydrated hydraulic artificial discs provide space and mobility between vertebral bones.
- Elastic – An elastic artificial disc, like a composite artificial disc, is made of two materials; however, an elastic artificial disc has a polycarbonate urethane core between two metal plates rather than a plastic core. The center core is “deformable,” which is designed to mimic the natural viscoelastic properties of the natural disc.
- Mechanical – A mechanical artificial disc is usually comprised of two articulating pieces, all of which are the same material (e.g. metal) or a composite metal and ceramic.
CERVICAL ARTIFICIAL DISCS
The BRYAN® Cervical Disc
The placement of the BRYAN® Cervical Disc was first reported in 2002.4 The placement of the BRYAN® Cervical Disc consists of a polycarbonate urethane nucleus that rests between two titanium alloy “shells.” Saline inside the nucleus adds compression to the disc. The metal parts of the BRYAN® device that interface with bone are porous to help integration. Because of its design, the BRYAN® Cervical Disc requires placement of the prosthesis precisely in its proper position. While the design is intriguing, the BRYAN® Cervical Disc is now obsolete.

The BRYAN® Cervical Disc
PRESTIGE® Cervical Disc
The PRESTIGE® Cervical Disc is made of a titanium ceramic composite and titanium carbide. The PRESTIGE® Cervical Disc has a “ball and trough” design, which means the upper part of the device has a rounded edge that moves in a divot in the bottom section of the artificial disc. This design and composition make the PRESTIGE® Cervical Disc highly durable and give the spine outstanding motion at one- and two-disc levels in the cervical spine. The endplates each have two low profile keels to help secure it to bone. Once placed, the artificial disc permits the spine to flex, extend, side bend, and rotate while maintaining alignment, height, and curvature of the natural spine. Of note, an earlier design called the PRESTIGE ST is now obsolete and has been replaced with the PRESTIGE LP. Patients continue to experience excellent results at least 10 years after artificial disc replacement surgery with the PRESTIGE® LP Cervical Disc.

PRESTIGE® Cervical Disc
Mobi-C® Cervical Disc
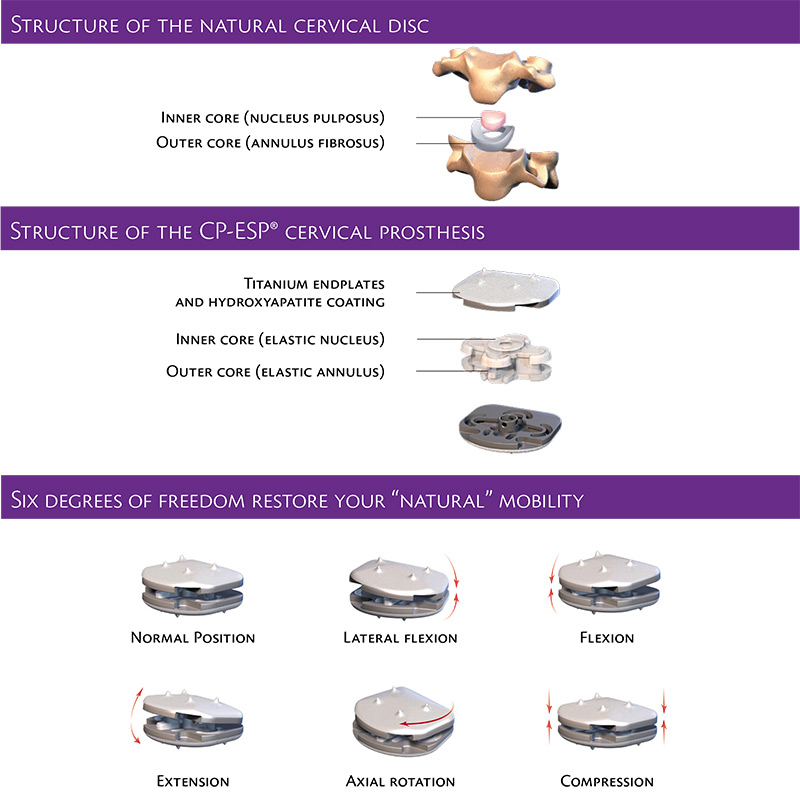
The upper and lower bone-facing endplates of the Mobi-C® Cervical Disc are made of a cobalt chromium molybdenum alloy. The endplates are coated with a fine layer of titanium and hydroxyapatite to promote integration with bone. A mobile, polyethylene bearing rests between the two endplates to give the device and the spine good range of motion.
Mobi-C® Cervical Disc
SECURE-C® Cervical Disc
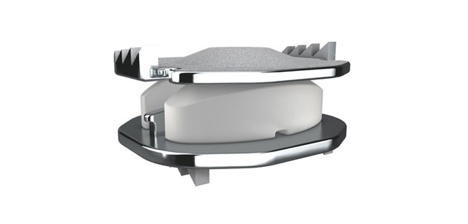
The SECURE-C® Cervical Disc is three separate pieces: a polyethylene inner core that is inserted between two cobalt chromium molybdenum alloy (metal) endplates. The specifications of the SECURE-C® Cervical Disc suggest that it permits up to ±15º motion in flexion-extension (nodding up and down) and up to ±10º motion in lateral bending (tilting the head from side to side).

SECURE-C® Cervical Disc
PCM® Cervical Disc
The PCM® Cervical Disc is two pieces: the upper portion is a cobalt chromium alloy (metal) endplate, while the lower metal endplate contains an integrated polyethylene spacer. The device has a titanium calcium phosphate coating to promote integration to bone. The PCM® Cervical Disc received premarket approval from the FDA in 2012, and like other cervical artificial discs, showed superior performance to spinal fusion surgery.6 It was FDA approved to be implanted adjacent to a fusion. It has since been removed from the market.

PCM® Cervical Disc
Prodisc-C® Cervical Disc
The Prodisc-C® Cervical Total Disc Replacement consists of two cobalt chromium molybdenum endplates and one ultra-high molecular weight polyethylene inlay. The inlay is technically separate from the endplate, but it locks into the lower metal such that they function as a single piece after installation. The upper endplate has a highly polished divot into which the plastic dome fits and moves. The metal surfaces are coated with a titanium plasma spray to help hold the artificial disc in place and promote bony growth.

Prodisc-C® Cervical Disc

Prodisc-C® Cervical Disc
Why Prodisc-C®?
Prodisc-C® Surgical Technique
Prodisc-C® Vivo
Composed of four components to maximize restoration of motion, the prodisc® C Vivo has been designed to replace a diseased and/or degenerated intervertebral disc in patients with symptomatic cervical disc disease (SCDD). Composed of four components utilizing the tried and tested ball and socket design, the prodisc® C Vivo resists shear forces, allows for flexion, extension, rotation and lateral bending, with secure teeth fixation providing high level stability
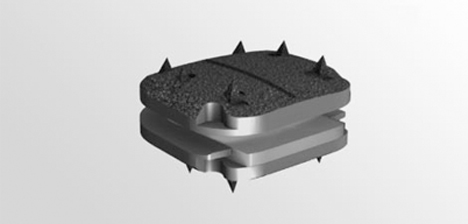
Prodisc-C® Vivo
Prodisc-C® SK
Similar to the prodisc® C Vivo, the prodisc® C SK is also low profile, allowing for multilevel application (“stacking” of the discs at adjacent spinal levels), which is necessary for many patients who have more than one damaged disc in their neck that requires replacement. Rather than secure teeth fixation to hold the device in place, the prodisc® C SK incorporates low profile “keels” for fixation.

Prodisc-C® SK
Prodisc-C® Nova
The prodisc C Nova implant has been designed to maintain the physiologic range of motion in the spine. The implant was developed using the clinically proven ball and socket concept used in joint replacement implants for over 40 years. The prodisc C Nova implant is composed of four components—two titanium alloy (TAN) endplates, a cobalt chrome alloy (CoCrMo) calotte insert, and an ultra-high molecular weight polyethylene (UHMWPE) inlay—and is inserted into the vertebral bodies en-bloc.

M6-C Artificial Cervical Disc
The M6-C artificial cervical disc has 4 components: Titanium endplates, a viscoelastic polymer nucleus, an ultra-high molecular polyethylene annulus, and sheath. The two outer titanium plates have fixation keels that anchor the artificial disc into the vertebral bone and are coated with a titanium plasma spray to help promote integration with bone. The sheath surrounds the artificial nucleus and artificial annulus, presumably to keep out bone and other debris. Like other artificial discs, the artificial nucleus and artificial annulus allow for good range of motion in the treated spine.
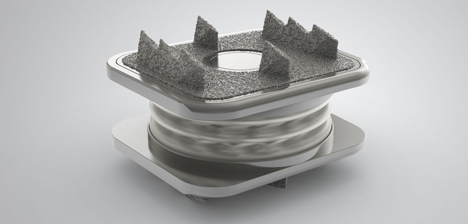
Prodisc-C® Nova
Simplify Cervical Artificial Disc
The Simplify® disc is comprised of 3 pieces in a semi-constrained design. This allows for 12 degrees of flexion and extension as well as lateral bending and axial rotation. It comes in various disc sizes, the lowest height is 4 mm. Interestingly, Simplify is made of MRI-safe materials, specifically polyetheretherketone (PEEK) and a mobile zirconia-toughened alumina ceramic core.
This means patients can have MRI studies after artificial disc replacement surgery, if needed. The endplates are serrated and have 2-3 “teeth” to help hold the device in place. As with other modern artificial discs, Simplify® is coated with titanium plasma spray on the faces of the device that contact bone. After 24 months, 97% of study participants were satisfied with Simplify® Cervical Artificial Disc treatment and 94% would have treatment again with the device, if necessary.

Simplify Cervical Artificial Disc
BAGUERA® C artificial cervical disc
BAGUERA® C is a prosthesis intended as a replacement for a cervical intervertebral disc. The BAGUERA® C range is indicated for patient suffering from symptomatic cervical disc disease (SCDD) affecting one level or two adjacent levels between C3 and C7. The guided mobile PE nucleus is designed to prevent excessive constraints on the facet joints. It allows 6 degrees of freedom. The sloping anatomical design of the plates optimizes the fit between the device and the disc space, and maximizes the endplate coverage. The titanium plates, coated with Diamond-Like-Carbon (DLC) reduce artefact under MRI for a better postoperative follow-up.
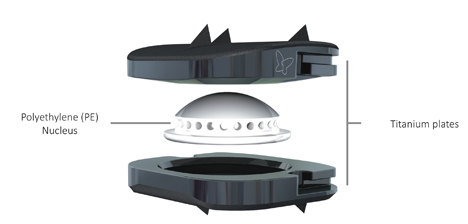
BAGUERA® C artificial cervical disc
LUMBAR ARTIFICIAL DISCS
CHARITÉ® Artificial Disc
The CHARITÉ® Artificial Disc was the first total disc replacement device to be developed and tested. The Type III version CHARITÉ® Artificial Disc has two highly polished metal endplates on either side of an ultra-high molecular weight polyethylene core.3 The endplates are porous so that bone can grow into and bond with the artificial disc. It was primarily used as a lumbar disc replacement and has since been removed from the market.

CHARITÉ® Artificial Disc
Prodisc-L® Lumbar Disc
The Prodisc-L® Total Lumbar Disc Replacement consists of two cobalt chrome alloy (metal) endplates and one ultra-high molecular weight polyethylene inlay. Each endplate has a single keel and has been plasma-sprayed with titanium to help promote bone integration. While tens of thousands of patients have received Prodisc-L® artificial discs at one vertebral disc level since its approval in 2005, the FDA approved the device to treat two-level disease in Spring 2020.

Prodisc-L® Lumbar Disc

Prodisc-L® Lumbar Disc
Prodisc-L® Surgical Technique
Why Prodisc-L®?
ACTIV-L
The activL Artificial Disc has two titanium-coated cobalt-chromium metal alloy endplates with a mobile ultra-high molecular weight polyethylene core. The plastic core is flat on the bottom and round on top, providing both structural support and range of motion. Relative to other artificial lumbar disc designs, the active-L Artificial Disc has the lowest height and the endplates come in a variety of sizes to fit individual patient anatomy. The active-L Artificial Disc can be used in patients who have one level of degenerative disc disease at either L4/L5 or L5/S1.

ACTIV-L
BAGUERA® L artificial lumbar disc
BAGUERA®L is the Lumbar artificial disc from SpineArt.
Benefits:
- Reduced MRI Artifact :The titanium plates reduce artifacts under the MRI and improve the postoperative control. The plates are coated with DIAMOLITH®.
- Shock Absorption :The shape of the inferior plate and the PE nucleus are designed to enable absorption of shocks and vibrations.
- Mobile of Fixed Nucleus :The BAGUERA®L concept allows the surgeon to choose the mobility of the nucleus intraoperatively, without changing the superior or inferior plates. The movement of the mobile nucleus is controlled to respect rotation movements.
- Primary Stability :The porous titanium coating as well as the 5 upper and 5 lower fins are designed for primary and secondary stability

BAGUERA® L artificial lumbar disc
LP-ESP Surgical Technique 3D Video
- For more information on Baguera-L lumbar disc replacement click here
- For more information on Baguera-C cervical disc replacement click here
- For more information on CP-ESP cervical disc replacement click here
- For more information on LP-ESP lumbar disc replacement click here
- For more information on Post-op protocol after lumbar disc replacement click here
- For more information on PRODISC-C VIVO cervical disc replacement click here
- For more information on PRODISC-L lumbar disc replacement click here